Advancing science. Improving connections.
We focus our research and development efforts on rare (affecting fewer than 200,000 patients in the U.S.) neuropsychiatric conditions.
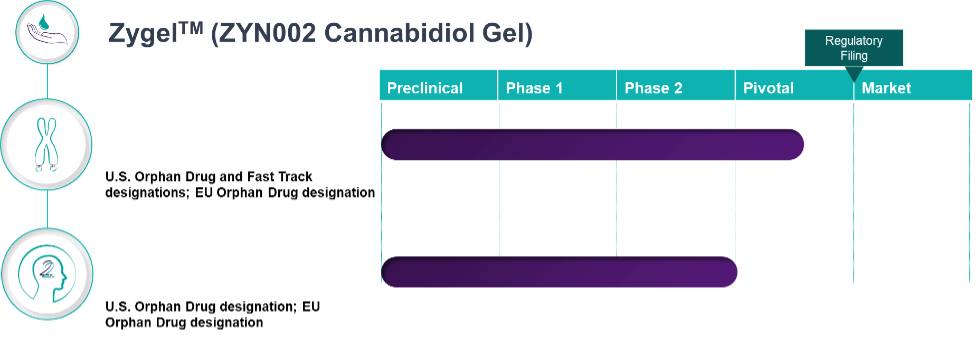
Our lead product candidate, ZygelTM is a cannabidiol gel currently being evaluated for Fragile X syndrome (FXS) and 22q11.2 deletion syndrome (22q).
Clinical Pipeline and Expected Milestones
Clinical Pipeline