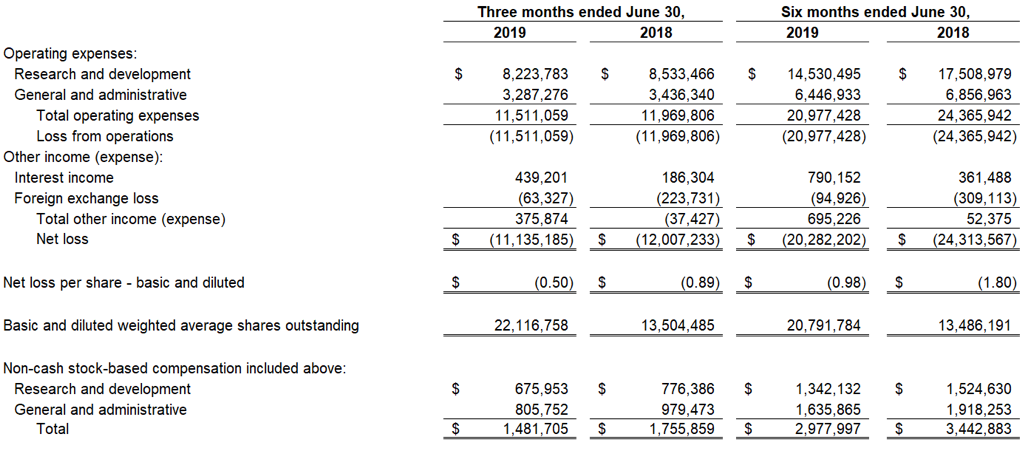
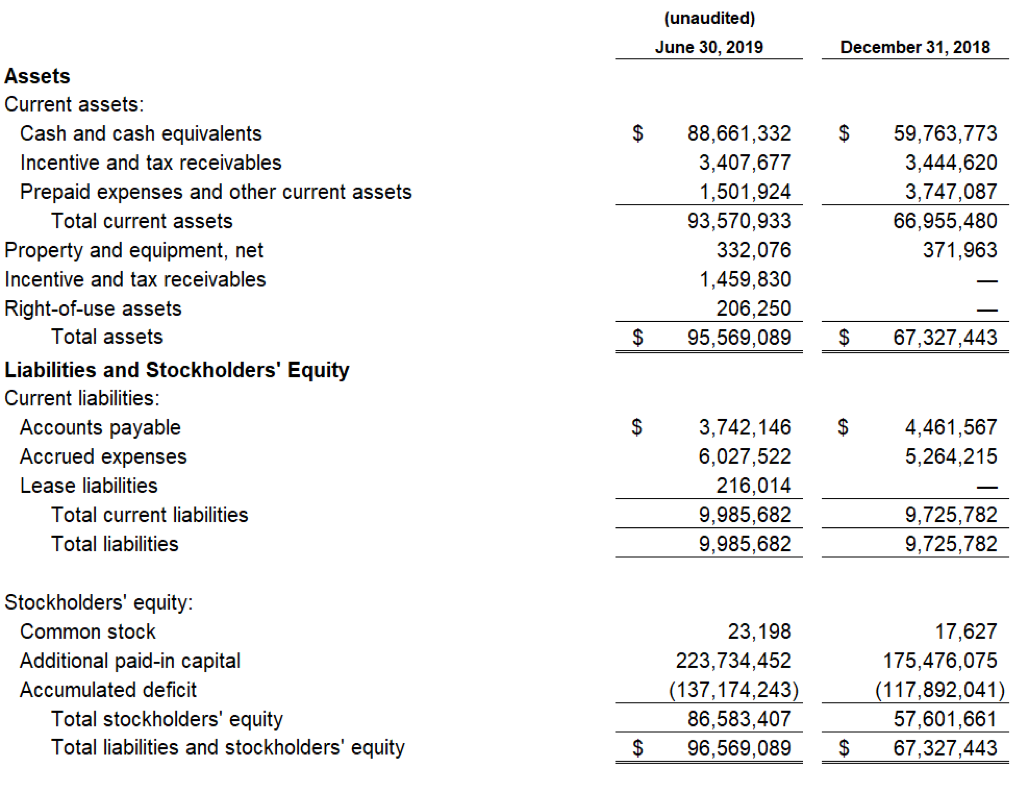
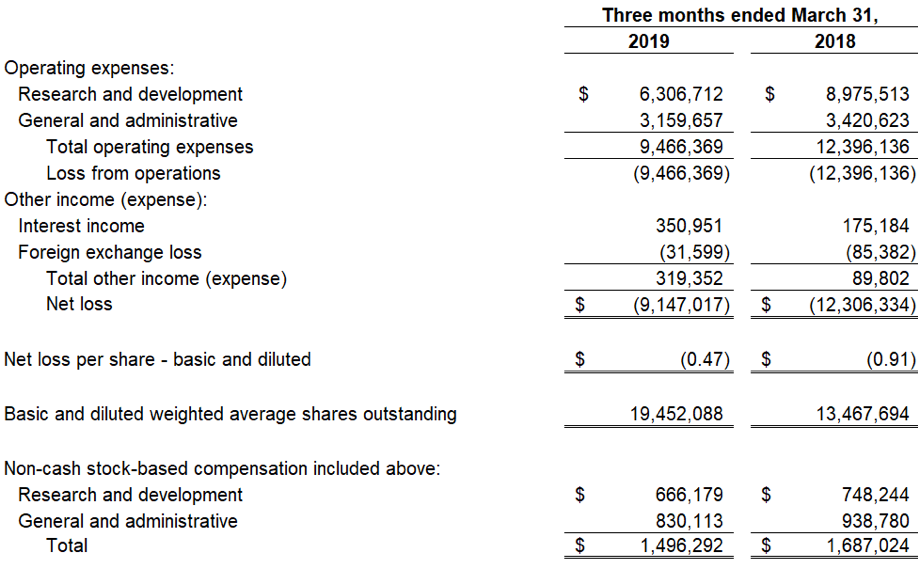
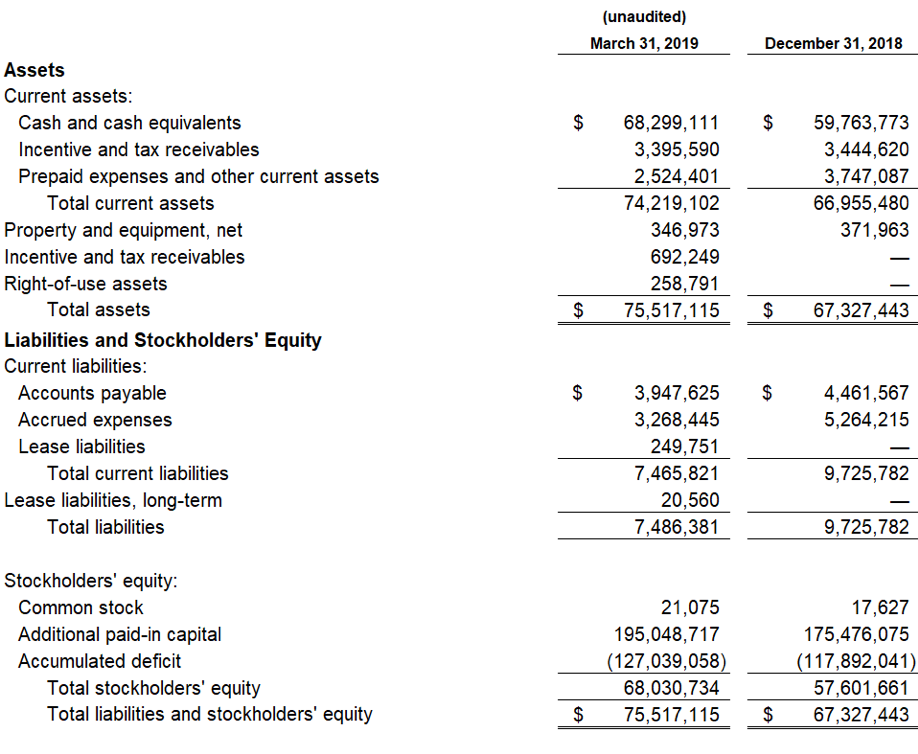
Devon, PA, August 6, 2019 — Zynerba Pharmaceuticals, Inc.(NASDAQ:ZYNE), the leader in innovative pharmaceutically-produced transdermal cannabinoid therapies for rare and near-rare neuropsychiatric disorders, today announced the publication of data from the Phase 2 FAB-C (Treatment of Fragile X Syndrome Anxiety and Behavioral Challenges with CBD) clinical trial. This trial evaluated ZYN002 cannabidiol (CBD) gel in pediatric and adolescent patients with Fragile X syndrome (FXS). The results of the trial have been published in a paper entitled, ‘A Phase 1/2, Open Label Assessment of the Safety, Tolerability, and Efficacy of Transdermal Cannabidiol (ZYN002) for the Treatment of Pediatric Fragile X Syndrome’ (Heussler, Helen; Cohen, Jonathan; Silove, Natalie, et al.) in the August 2ndonline edition of Journal of Neurodevelopmental Disorders. These data were recently presented at the 2019 Annual Meeting of the American Psychiatric Association (APA).
This new publication can be accessed at the following link: https://rdcu.be/bMTnd.
“Fragile X is a family diagnosis, impacting not only the diagnosed child but also the entire family unit,” said Honey Heussler, MBBS, FRACP, MRCPCH, PGCAP, DM, Associate Professor, University of Queensland and Medical Director Child Development, Children’s Health Queensland, and an investigator in the FAB-C study. “Children with Fragile X syndrome exhibit a number of developmental and behavioral symptoms including anxiety, social avoidance, hyperactivity, and socially unresponsive behaviors that significantly impact the family, and the child’s capacity to interact with them, their peers, and care providers. My hope is that if we can successfully treat these symptoms, we can enhance the child’s ability to engage and interact. In that regard, these open label data are promising, and are an important step in the development of ZYN002. I look forward to the results of the ongoing double blind, placebo-controlled CONNECT-FX study.”
In summary, the authors suggest that the results from this open-label trial indicate that ZYN002 may be an effective treatment for many behavioral and emotional symptoms associated with FXS although the study’s findings are limited by the open label design and small sample size. Given the lack of medications approved for the treatment of FXS, these open-label study findings highlight the urgent need for randomized, controlled, clinical trials to further assess the safety and efficacy of ZYN002 for FXS symptoms ranging from social avoidance, irritability, social unresponsiveness/lethargy and stereotypy, to anxiety. To that end, enrollment is ongoing in CONNECT-FX, a multi-national, randomized, double blind placebo controlled pivotal clinical trial of Zygel (ZYN002) in FXS.
The FAB-C study enrolled 20 male and female pediatric patients with FXS, six through 17 years of age at screening, with a diagnosis of FXS confirmed through molecular documentation of FMR1 full mutation. Eligible patients were provided 12 weeks of ZYN002 as an adjunct to their existing treatments (a six-week titration period, followed by six-week maintenance period) at three dose levels: once daily 50 mg dose, twice daily 50 mg dose (100 mg total), or twice daily 125 mg dose (250 mg total). During the six-week titration period, patients could be titrated up to 250 mg daily. Safety and tolerability were assessed bi-weekly through physical/neurological exam, vital sign collection, 12-lead electrocardiograms (ECGs), a Modified Suicidality Checklist, safety laboratory tests, pregnancy tests, urinalysis, and monitoring for adverse events (AEs). In addition, several measures assessing mood, behavior, and functioning were selected, including the Anxiety, Depression, and Mood Scale (ADAMS), the Aberrant Behavior Checklist – Community (ABC-C) for FXS (ABC-CFXS), the Pediatric Anxiety Rating Scale (PARS-R), the Pediatric Quality of Life Inventory (PedsQL™), three Visual Analogue Scales (VAS) (anxiety, hyperactivity/impulsivity, and tantrum/mood lability), and the Clinical Global Impression Scale – Severity (CGI-S) and Improvement (CGI-I).
Results from the efficacy analyses all converge to suggest a pattern of clinical improvement in a range of key parent and clinician rated emotional and behavioral symptoms of FXS:
- Patients experienced significant 12-week improvement over baseline scores for the majority of study efficacy endpoints, including the change from screening to Week 12 in the ADAMS Total score, 4 out of the 5 ADAMS subscale scores, the six subscales of the ABC-CFXS, PARS-R, PedsQL, VAS (anxiety, hyperactivity/impulsivity, and tantrum/mood lability), and CGI-I;
- The greatest emotional improvement following treatment was observed for Anxiety (ADAMS, PARS-R, VAS (anxiety); d = 0.98 to 1.70), while behavioral improvements were most pronounced in the domains of Social Avoidance (ABC-CFXS; d = 1.00) and Stereotypy (ABC-CFXS; d = 0.99);
- Importantly, observed improvements were generally greater than those demonstrated for placebo in prior controlled clinical trials in FXS; and
- Across assessments, there was consensus in improvement in both internalizing (e.g., anxiety, social avoidance) and externalizing symptoms (e.g., irritability) over the course of treatment.
ZYN002 was well tolerated in this study:
- The majority of AEs were mild in severity and resolved by the end of the 12-week treatment period with no dose adjustment;
- No serious adverse events (SAEs) were reported;
- There were no clinically meaningful trends in laboratory values (including testosterone levels), except for an increase in eosinophil count at Day 83 by a patient reporting an AE of moderate rash (patient completed the study). A repeat blood collection done one month later on this patient showed a decreased but slightly above normal eosinophil count; and
- There were no clinically significant changes in liver function tests.
About Zygel™ (ZYN002)
Zygel (CBD gel) is the first and only pharmaceutically-manufactured CBD formulated as a patent-protected permeation-enhanced clear gel, designed to provide controlled drug delivery into the bloodstream transdermally (i.e. through the skin). Recent studies suggest that Fragile X Syndrome (FXS) and other neuropsychiatric conditions may be associated with a disruption in the endocannabinoid (EC) system. Clinical and anecdotal data suggest that CBD may modulate the EC system and improve certain core social and behavioral symptoms, including social avoidance (prefers isolation from others, prefers solitary activities, avoids new social activities), irritability (aggressive to others, tantrums/outbursts, and stubbornness), social unresponsiveness/lethargy (lack of attention/interaction, inactive/lack of movement and can resist physical contact), and anxiety.
Zygel has been designated a Fast Track development program by the U.S. Food and Drug Administration for treatment of behavioral symptoms of FXS. Enrollment is ongoing in CONNECT-FX, a multi-national, randomized, double blind placebo controlled pivotal clinical trial of Zygel in FXS (https://www.connectfxtrial.com/); topline data are expected in the first half of 2020. Additionally, Zynerba expects topline data from its Phase 2 open label BELIEVE 1 trial of Zygel in developmental and epileptic encephalopathies (DEE) in September 2019. Zynerba has also initiated the Phase 2 BRIGHT trial in autism spectrum disorder and the Phase 2 INSPIRE trial in 22q11.2 deletion syndrome, with data expected from both studies in the first half of 2020.
About Zynerba Pharmaceuticals, Inc.
Zynerba Pharmaceuticals is the leader in pharmaceutically-produced transdermal cannabinoid therapies for rare and near-rare neuropsychiatric disorders. We are committed to improving the lives of patients and their families living with severe, chronic health conditions including Fragile X syndrome, autism spectrum disorder, 22q11.2 deletion syndrome, and a heterogeneous group of rare and ultra-rare epilepsies known as developmental and epileptic encephalopathies. Learn more at www.zynerba.com and follow us on Twitter at @ZynerbaPharma.
Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from the Company’s current expectations. For example, there can be no guarantee that the Company will obtain approval for Zygel from the U.S. Food and Drug Administration (FDA) or foreign regulatory authorities; even if Zygel is approved, the Company may not be able to obtain the label claims that it is seeking from the FDA. Management’s expectations and, therefore, any forward-looking statements in this press release could also be affected by risks and uncertainties relating to a number of other factors, including the following: the Company’s cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the Company’s ability to obtain additional funding to support its clinical development programs; the results, cost and timing of the Company’s clinical development programs, including any delays to such clinical trials relating to enrollment or site initiation; clinical results for the Company’s product candidates may not be replicated or continue to occur in additional trials and may not otherwise support further development in a specified indication or at all; actions or advice of the FDA and foreign regulatory agencies may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for additional clinical trials; the Company’s ability to obtain and maintain regulatory approval for its product candidates, and the labeling under any such approval; the Company’s reliance on third parties to assist in conducting pre-clinical and clinical trials for its product candidates; delays, interruptions or failures in the manufacture and supply of the Company’s product candidates the Company’s ability to commercialize its product candidates; the size and growth potential of the markets for the Company’s product candidates, and the Company’s ability to service those markets; the Company’s ability to develop sales and marketing capabilities, whether alone or with potential future collaborators; the rate and degree of market acceptance of the Company’s product candidates; and the Company’s expectations regarding its ability to obtain and adequately maintain sufficient intellectual property protection for its product candidates. This list is not exhaustive and these and other risks are described in the Company’s periodic reports, including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the Securities and Exchange Commission and available at www.sec.gov. Any forward-looking statements that the Company makes in this press release speak only as of the date of this press release. The Company assumes no obligation to update forward-looking statements whether as a result of new information, future events or otherwise, after the date of this press release.
Zynerba Contact
William Roberts, Vice President, Investor Relations and Corporate Communications
Zynerba Pharmaceuticals
484.581.7489
robertsw@zynerba.com