6 Aug 19
Zynerba Pharmaceuticals Reports Second Quarter 2019 Financial Results and Operational Highlights
Devon, PA, Aug. 06, 2019 — Zynerba Pharmaceuticals, Inc. (NASDAQ:ZYNE), the leader in innovative pharmaceutically-produced transdermal cannabinoid therapies for rare and near-rare neuropsychiatric disorders, today reported financial results for the second quarter ended June 30, 2019 and provided an overview of recent operational highlights.
“The past few months have been very productive for our team,” said Armando Anido, Chairman and Chief Executive Officer of Zynerba. “We initiated new studies in autism spectrum disorder and 22q11.2 deletion syndrome, obtained Fast Track Designation for Zygel™ in FXS, enhanced our senior management team with two excellent additions in Medical and Regulatory, received an important new patent for CBD, and were added to the Russell 2000® and 3000® indices. We also extended our cash runway into the second half of 2021 through the addition of $27.0 million in cash from our ATM in the second quarter and a positive decision from the Australian government that will provide us access to an incremental $7.0 to $9.0 million in research and development cash credits. This all sets the stage for the next 12 months to be potentially transformational as we report out on our FXS pivotal trial, and our Phase 2 trials in DEE, ASD and 22q.”
Continued Anido, “Regarding the CONNECT-FX study, we believe that pivotal data will now be available in the first half of 2020. We are thrilled with the interest in this study by families who have children with Fragile X syndrome, our investigators, and our advocacy partners. The study design includes specific entrance criteria that have resulted in a higher than predicted screen failure rate. Importantly, these entrance criteria have resulted in an enrolled population with more severe behavioral symptoms than the FAB-C study population. We believe this will enhance the study’s ability to demonstrate a strong signal of activity and minimize response variability.”
Second Quarter 2019 and Recent Highlights
Zygel in Fragile X Syndrome (FXS)
Fragile X Syndrome Pivotal Data Expected in the First Half of 2020
Enrollment is progressing in CONNECT-FX, a pivotal, multi-national, randomized, double blind, placebo-controlled trial evaluating the efficacy and safety of Zygel (ZYN002 CBD gel) in treating common behavioral symptoms of FXS in three through 17-year old patients with FXS. Clinical investigative sites are enrolling patients in the United States, Australia, and New Zealand. The Company expects to report top line data in the first half of 2020. If the data are positive, the Company expects to submit its New Drug Application (NDA) for Zygel in FXS to the U.S. Food and Drug Administration (FDA) in the second half of 2020, with potential approval by mid-year 2021.
Patients Enrolling Into CONNECT-FX Open Label Extension
Upon completion of the double blind placebo controlled portion of the CONNECT-FX study, patients are eligible to enroll into a 12-month extension study. At this point, all patients who have completed the double-blind phase of the study have enrolled into the extension phase.
Received Fast Track Designation for Zygel for Treatment of Behavioral Symptoms Associated with FXS
FDA’s Fast Track program is designed to facilitate the development of drugs intended to treat serious conditions and fill unmet medical needs and can lead to expedited review by FDA in order to get new important drugs to the patient earlier. This designation is in addition to the previously received Orphan Drug designation.
Zygel in Developmental and Epileptic Encephalopathies (DEE)
Topline Results Expected in September 2019
The Company expects to announce topline data from BELIEVE 1, an open label multi-dose Phase 2 clinical trial evaluating the efficacy and safety of Zygel in children and adolescents (three through 17 years) with various DEEs, in September 2019. The primary efficacy assessment is reduction in seizure frequency at week 26 compared to baseline.
Zygel in Autism Spectrum Disorder (ASD)
Enrollment Ongoing in Phase 2 Open Label Trial of Zygel in ASD; Data Expected in the First Half of 2020
The Company initiated the Phase 2 BRIGHT trial in March 2019 to assess the safety, tolerability and efficacy of Zygel for the treatment of child and adolescent patients with ASD. The 14-week trial is designed to evaluate the efficacy and safety of Zygel in approximately 36 children and adolescents (ages four through 17) with ASD as confirmed by DSM-5 diagnostic criteria for ASD. The efficacy assessments include the Aberrant Behavior Checklist, Parent Rated Anxiety Scale – Autism Spectrum Disorder, Autism Impact Measure, and Clinical Global Impression – Severity and Improvement. Zynerba expects to present topline data from this study in the first half of 2020.
Announced Receipt of New U.S. Patent for Treatment of ASD with Cannabidiol (CBD)
The U.S. Patent and Trademark Office issued U.S. Patent No. 10,314,792 titled “Treatment of Autism Spectrum Disorder with Cannabidiol” which includes claims directed to methods of treating autism spectrum disorder by administering a therapeutically effective amount of synthetic cannabidiol. This new patent expires in 2038 and is part of an expanding intellectual property portfolio covering Zygel.
Zygel in 22q11.2 Deletion Syndrome (22q)
Announced Initiation of Phase 2 Open Label Trial of Zygel in 22q; Data Expected in the First Half of 2020
The Company initiated the 14-week Phase 2 INSPIRE trial in May 2019 to evaluate the safety, tolerability and efficacy of Zygel in approximately 20 children and adolescents (ages six through 17) with genetically-confirmed 22q. The efficacy assessments include the Aberrant Behavior Checklist-Community (ABC-C), the Anxiety, Depression and Mood Scale (ADAMS), the Qualitative Caregiver Reported Behavioral Problem Survey, and Clinical Global Impression – Severity and Improvement.
Corporate
Advance Overseas Finding Approved by AusIndustry; Expected to Generate $7.0 to $9.0 Million
In July 2019, the Australian government’s Department of Industry, Innovation and Science (AusIndustry) responded to an Advance Overseas Finding (AOF) application submitted by Zynerba that will allow certain research and development expenses incurred with respect to Zygel outside of Australia to be eligible for the Australian research and development tax incentive program. As a result of this finding, the Company is eligible to receive a cash refund from the Australian Taxation Office for the qualifying research and development costs expended outside of Australia in 2018, 2019 and 2020. Management believes that this decision will generate an incremental $7.0 to $9.0 million in cash tax credits over the next 18 to 24 months.
Enhanced Senior Management Team
Joseph Palumbo, M.D. recently joined the Company as Chief Medical Officer. He has over 30 years of experience in areas including neurology and neuro-degeneration, psychiatry and behavioral medicine, cognition, and orphan conditions in companies and institutions including Mitsubishi Tanabe, Johnson & Johnson/Janssen, Sanofi-Synthelabo, Cephalon, Yale, Cornell, and the University of Pennsylvania. Dr. Palumbo is Board Certified in Psychiatry and has worked on the approval of therapeutics for neuropsychiatric disorders including Radicava® (edaravone), Risperdal® (risperidone), and Invega®(paliperidone)1.
“I believe that the critical clinical research that Zynerba is performing has the potential to change treatment paradigms in neuropsychiatric disorders,” commented Dr. Palumbo. “If the ongoing studies are successful, and if Zygel is approved, there is real hope to see dramatic improvement in the daily lives of the families affected by conditions such as Fragile X. I’m very excited to join a seasoned, international team of individuals who have experienced success in all aspects of pharmaceutical drug development in the past, and I look forward to being part of Zynerba’s continued growth and evolution.”
Pam Swiggard, M.S. has joined Zynerba as Vice President, Regulatory Affairs and Quality. She is a senior pharmaceutical executive with nearly 30 years of experience in areas including clinical trial management, regulatory affairs, and quality with companies including Fibrocell Science, Inc., Trevena Inc., Endo Pharmaceuticals, and Wyeth Pharmaceuticals.
Company Added to Russell Indexes
The Company was added as a member of the U.S. all-cap Russell 3000® and small-cap Russell 2000® Indexes at the conclusion of the 2019 Russell indexes annual reconstitution at the open of the U.S. markets on July 1, 2019.
_____________
1 Radicava is a trademark of Mitsubishi Tanabe Pharma Corporation. Risperdal and Invega are registered trademarks of Janssen Pharmaceuticals, Inc.
Second Quarter 2019 Financial Results
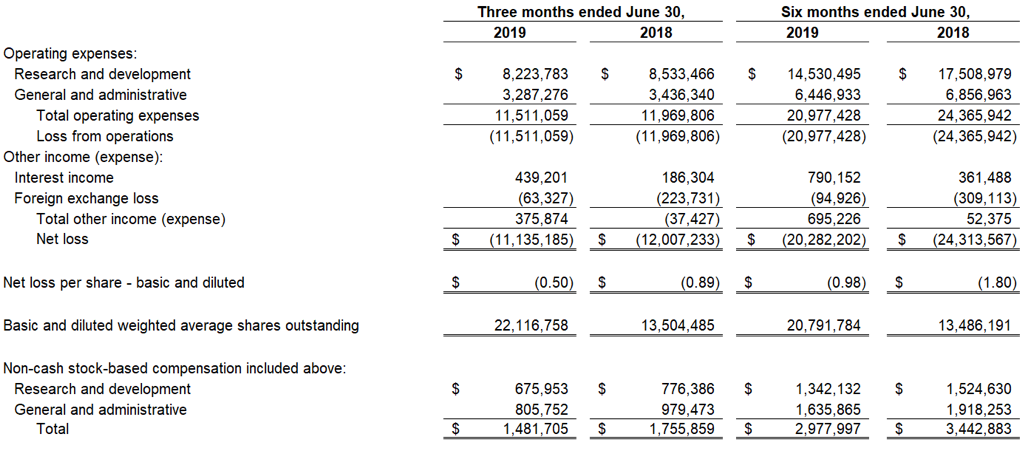
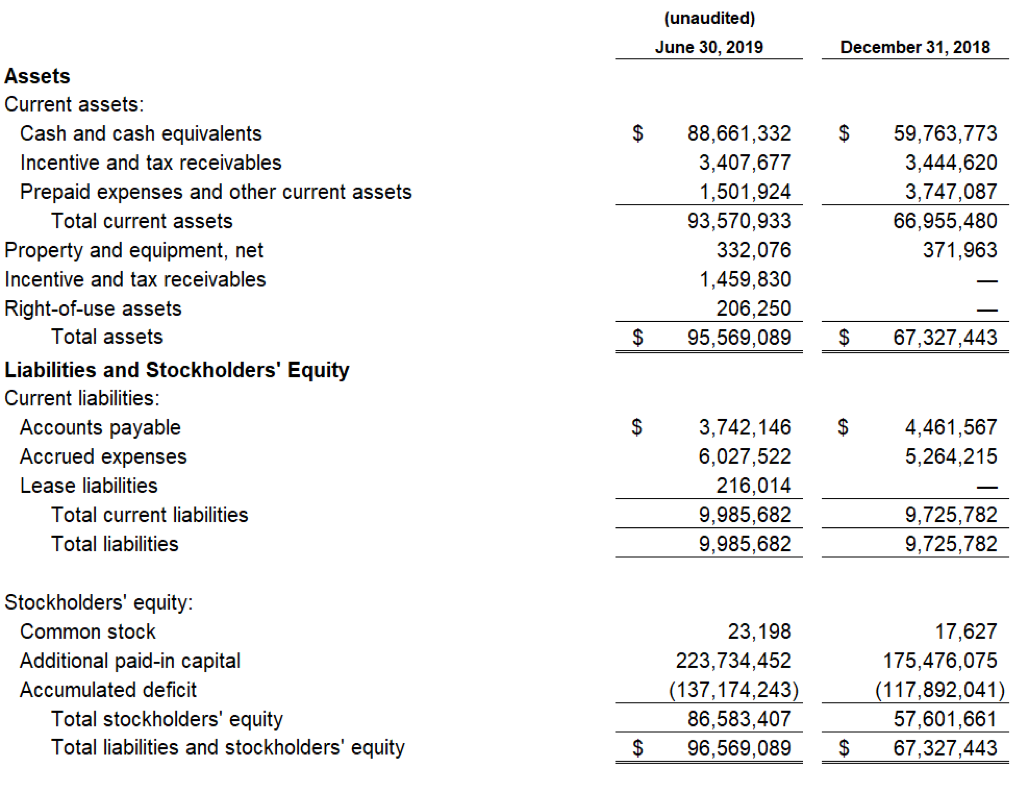
As of June 30, 2019, cash and cash equivalents were $88.7 million, compared to $59.8 million as of December 31, 2018. Included in this cash and cash equivalents position is $27.0 million in net proceeds, after deducting commissions and offering expenses, from 2,082,031 shares sold and issued at a weighted average selling price of $13.50 per share during the second quarter of 2019 under an Open Market Sales Agreement, or “at-the-market” (ATM) offering program, with Jefferies LLC.
Research and development expenses for the second quarter of 2019 were $8.2 million, including stock-based compensation of $0.7 million. General and administrative expenses for the second quarter of 2019 were $3.3 million, including stock-based compensation expense of $0.8 million. The net loss for the second quarter of 2019 was $11.1 million with basic and diluted net loss per share of $(0.50).
Financial Outlook
The Company’s cash and cash equivalent position as of June 30, 2019 was $88.7 million. Management believes that the cash and cash equivalent position including proceeds anticipated from the Australian AOF is sufficient to fund operations and capital requirements beyond the expected NDA submission and potential approval of Zygel in FXS and into the second half of 2021.
About Zynerba Pharmaceuticals, Inc.
Zynerba Pharmaceuticals is the leader in pharmaceutically-produced transdermal cannabinoid therapies for rare and near-rare neuropsychiatric disorders. We are committed to improving the lives of patients and their families living with severe, chronic health conditions including Fragile X syndrome, autism spectrum disorder, 22q11.2 deletion syndrome, and a heterogeneous group of rare and ultra-rare epilepsies known as developmental and epileptic encephalopathies. Learn more at www.zynerba.com and follow us on Twitter at @ZynerbaPharma.
Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from the Company’s current expectations. Management’s expectations and, therefore, any forward-looking statements in this press release could also be affected by risks and uncertainties relating to a number of other factors, including the following: the Company’s cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the Company’s ability to obtain additional funding to support its clinical development programs; the results, cost and timing of the Company’s clinical development programs, including any delays to such clinical trials relating to enrollment or site initiation; clinical results for the Company’s product candidates may not be replicated or continue to occur in additional trials and may not otherwise support further development in a specified indication or at all; actions or advice of the U.S. Food and Drug Administrationand foreign regulatory agencies may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for additional clinical trials; the Company’s ability to obtain and maintain regulatory approval for its product candidates, and the labeling under any such approval; the Company’s reliance on third parties to assist in conducting pre-clinical and clinical trials for its product candidates; delays, interruptions or failures in the manufacture and supply of the Company’s product candidates the Company’s ability to commercialize its product candidates; the size and growth potential of the markets for the Company’s product candidates, and the Company’s ability to service those markets; the Company’s ability to develop sales and marketing capabilities, whether alone or with potential future collaborators; the rate and degree of market acceptance of the Company’s product candidates; and the Company’s expectations regarding its ability to obtain and adequately maintain sufficient intellectual property protection for its product candidates. This list is not exhaustive and these and other risks are described in the Company’s periodic reports, including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the Securities and Exchange Commissionand available at www.sec.gov. Any forward-looking statements that the Company makes in this press release speak only as of the date of this press release. The Company assumes no obligation to update forward-looking statements whether as a result of new information, future events or otherwise, after the date of this press release.
ZYNERBA PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(unaudited)
ZYNERBA PHARMACEUTICALS, INC.
CONSOLIDATED BALANCE SHEETS
Zynerba Contacts
Jim Fickenscher, CFO and VP Corporate Development
Zynerba Pharmaceuticals
484.581.7483
fickenscherj@zynerba.com
Will Roberts, VP Investor Relations and Corporate Communications
Zynerba Pharmaceuticals
484.581.7489
robertsw@zynerba.com

